|
What is pH
pH is a scale measuring the acidity or alkalinity of a solution.
The scientific definition is 'the negative logarithm of the Hydrogen ion concentration'. The pH scale is a measurement
of the concentration of acid or base a substance contains and is calculated by determining the concentration of hydrogen
ions (H+).
High hydrogen ion amounts indicate high acid concentration,
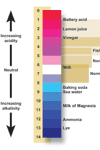
low hydrogen ion amounts indicates a basic or alkaline concentration. The pH scale runs from 0 to 14 with 0 being the
most acidic, 7 neutral, and 14 being the most alkaline or basic. It is a logarithmic scale, based on powers of 10, so
that 1 pH unit change equals a 10 fold change in H+ ion concentration! A pH of 6 is ten times more acidic than a pH of 7.
The sanitizing ability of chlorine is achieved by it turning into hypoclorous acid. pH affects the efficiency of chlorine
by determining the amount of hypoclorous acid (free available chlorine) that is formed.
- At pH 6.5, 90% of the chlorine will be hypochlorous acid
- At pH 7.5, 50% of the chlorine will be hypochlorous acid
- At pH 8.0, 20% of the chlorine will be hypochlorous acid
Unfortunately you cannot run your water at pH 6.5 as it would be very acidic and corrode any metals in your system. The
number one cause of heater failure is corrosion due to low pH. As any metals corrode away, the resulting metal oxides can
cause surface staining, and under some conditions even tint your hair. Lower pH is also far from the human body's pH of 7.4
and can be uncomfortable to soak in.
At a high pH, the water can make your eyes sting and possibly give you a sore throat. A high pH can also cause scale to form.
This is because at a pH of around 8.0, the calcium in the water combines with carbonates in the water to form calcium carbonate.
Calcium carbonate can form scale deposits on your spas surface or in the plumbing system. It can also form into tiny particles
and float around in the water giving it a cloudy, turbid appearance.
The compromise is pH of 7.2 to 7.6, preferably the midpoint of 7.4. Remember, if your pH drifts too high, it will require more
chlorine to get adequate disinfection.
pH can be determined in several ways. pH meters electronically measure the ion concentration, giving pH in a digital readout.
pH indicators are substances obtained from plant material that change color depending on the degree of acid or base of the
substance it is mixed with.
A few drops of indicator is added to a solution of the substance to be measured. The color the indicator changes to is matched
to a color chart which matches the color of the indicator to the corresponding pH. Some indicators have a wide range of color
changes from acid to base.
Others are specific to a small range with in the pH scale, say 2.5 to 5.0 pH. Red cabbage juice is a common indicator you might
be familiar with. Red cabbage juice has a color range from bright pink for a strong acid to yellow for a strong base. Other
indicators, such as litmus, indicate if a substance is acid or base, not the degree of acid or base.
Indicators can be soaked into papers, making indicator paper for specific pH ranges. Indicator papers can be dipped into
substances, matching the color change of the paper to a pH color chart for the indicator.
|
|
More Links:

See Our Video Archive for more ...

View our F.A.Q.section

Site Map

|